CFDA Certification
All the certification organizations, both governmental and non-governmental, share a common goal: the health and safety of human patients. Since people are basically the same all over the world, state-of-the-art medical devices will need to perform the same functions to the same quality level, no matter where they are operating.
Like the U.S. FDA, China divides medical devices into three classes according to risks to the patient. The CFDA/NMPA requires higher-risk devices to undergo tests in its own laboratories or by laboratories certified by the agency. Most imaging-related products are in the lower-risk categories, though.
The Chinese authority’s standards for good manufacturing practices are very similar to ISO 13485, so once you gain ISO certification, you could pass that information along to China. Subsequently, CFDA/NMPA auditors will review the ISO certification against the Chinese requirements.
When applying for Class II and Class III medical electrical equipment registration, the product must be tested by a CFDA-approved laboratory. It’s very difficult to get the CFDA certificate, in China less than 10 local suppliers own a real CFDA certificate, so we are your qualified supplier. Based on the CFDA certificate, the FSC certificate is also available.
CE Certiification
CE certificate is a system introduced to gain international competitiveness by integrating it into one market for the free sale and distribution of goods, finance, people and services that satisfy EHSR(Essential Health & Safety requirements) in the European Union (EU) market. Therefore, it is mandatory to mark CE on the product.
After a required technical document including a test report is submitted to the certification authority, Notified Body will issue the certificate after determining its suitability. After CE certificate is issued, it can mark CE on the product.
The machinery standard is based on the Machinery Directive 2006/42/EC and is applied EN ISO 12100, EN ISO 13849, and EN IEC 60204-1. In addition, if there is an "Harmonized Standard" corresponding to each machinery, that standard will be applied. And our team is now developing the next generation product for approval under the EU Medical Device Regulation 2017/745 (MDR).

VCSEL Tech
Since 2014, we first adopted VCSEL on hair removal applications and got patent from US and Europe. This is a revolutionary breakthrough in this filed, it’s replacing 60% market of the LD bar during the past 10 years.
Meanwhile, we adopt the VCSEL tech on all our production line. Including Alex755 Hair removal, Q-switched laser system for Tattoo removal, Laser therapy, Laser slimming etc. Each time we launch any new product, it’s a new trend in the market. With such cutting edge tech development, we are ahead of other competitors 3-5 years. Our team is more like a “Scientist” group, engaging in inventing the developing the best tech and best device for all clients.
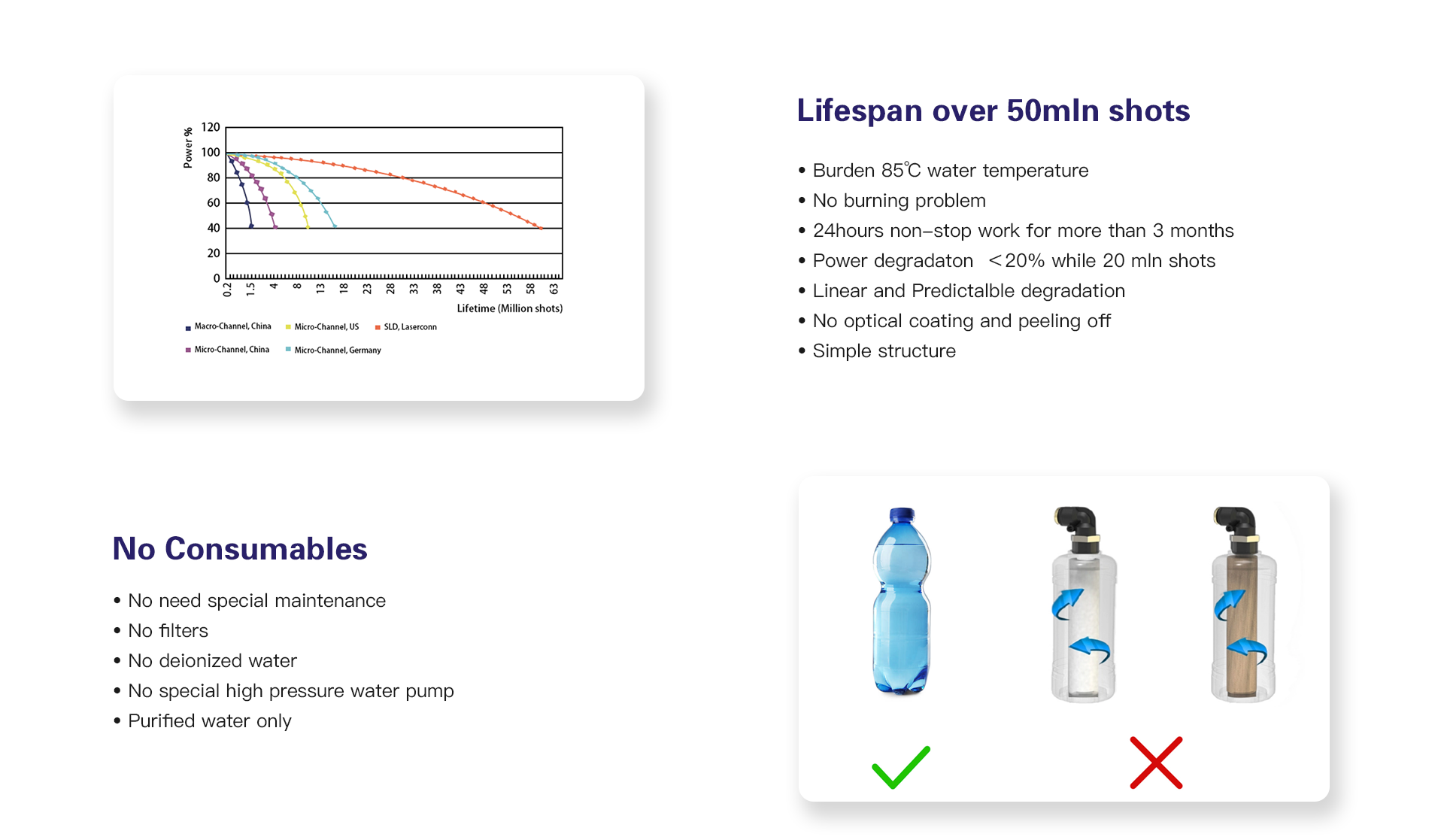
VCSEL is short for Vertical cavity surface emitting laser diode, with a big surface for emitting and heat spreading, it can burden temperature over 85℃. Thus it's quite stable.

Technical Specifications
| Milestone Standard | Parameter |
|
|
Wavelength 810nm Laser Power 800W or 1200W Pulse Width 4~160ms Frequency 1~10Hz Fluence 0~100J/cm2 Skin Cooling -10 to 5℃ Spot size 14x14mm LCD Screen 8.4" LCD Input VAC 110/220VAC / 50-60Hz Dimension 34×39×105cm Weight 40KG |
| Milestone Smart | Parameter |
|
|
Wavelength 810nm Laser Power 800W or 1200W Pulse Width 4~160ms Frequency 1~10Hz Fluence 0~100J/cm2 Skin Cooling -10 to 5℃ Spot size 14x14mm LCD Screen 8.4" LCD Input VAC 110/220VAC / 50-60Hz Dimension 50×35×20cm Weight 14KG |


